Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19 - Nature.com
8/22/2022 12:00:00 AM3 years 6 months ago
In April 2021, Eli Lilly voluntarily asked the FDA to revoke the Emergency Use Authorization for the monoclonal antibody bamlanivimab due to reduced susceptibility in vitro to SARS-CoV-2 variants, not for safety. In this work, authors carry out a placebo-cont…
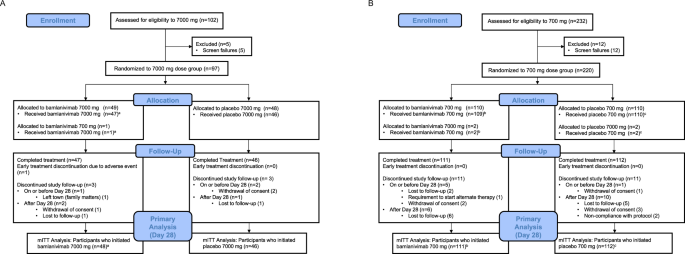
Trial design and oversight The ACTIV-2/A5401 study is an ongoing multicenter phase 2/3 adaptive platform randomized controlled trial for the evaluation of therapeutics for early COVID-19 in non-hosp… [+17432 chars]
full article...